Prix SFA 2021

CONGRÈS ANGIOGENÈSE – 9ÈME ÉDITION
1 septembre 2021
Lettre de la Présidente
21 mars 2022Prix de la meilleure communication orale

IEMN and LIMMS/IIS
SMMiL-E project
Lille, France
After an engineering degree at Ecole Centrale de Lille, I joined Fabrice SONCIN’s team for my PhD. Our lab is a multidisciplinary environment where I can apply my engineering skills to biological questions. Microfluidic devices are a turning point to answer complex biological questions. They aim at bridging the gap between too simple 2D in-vitro assays and costly, time consuming, and species-specific animal models. Highly efficient and simple methods for creating structurally sound blood vessels-on-chips (VoC) are needed in order to scale-up 3D screening assays of vascular functions.
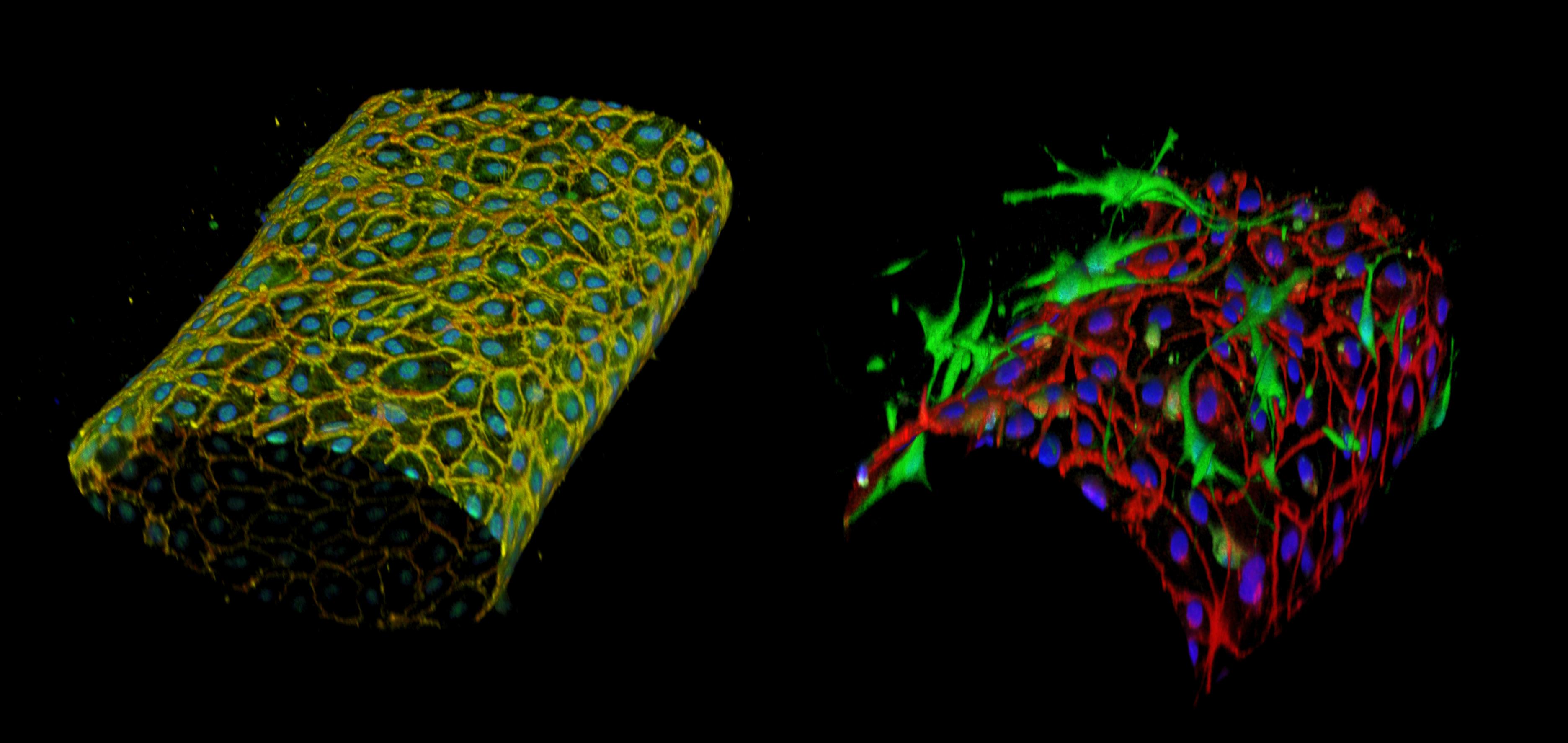
My project aims a creating VoC using the vascular finger patterning technique. We created blood vessels composed of two concentric, distinct, and closely positioned layers of human endothelial and perivascular cells arranged around the hollow lumen. This allowed for the formation of structurally correct blood vessels-on-chips which could be constituted, at will, of either only endothelial cells or of both cell types. The established vessels showed a tight vascular barrier and were fully reactive to permeability and to inflammatory factors. The design allowed for the establishment and testing of several tens of blood vessels simultaneously in a standard multi-well format suitable for high-throughput drug screening.


Molecular control of the neurovascular development
Centre Interdisciplinaire de Recherche en Biologie (CIRB)
UMR7241/INSERM U1050
Collège de France
Paris, France
Intra-nervous vascularization (INV) of peripheral nerves is important for their development, homeostasis and regeneration but remains largely poorly described. In the mouse, I have found that INV formation begins around embryonic day E16. Schwann cells and myelin appear to have a crucial role in the control of INV formation during development as their ablation provokes a disrupted and immature INV and an hypervascularisation of the sciatic nerve. The guidance molecule Netrin-1 and the receptor UNC5B could have a pro-angiogenic role during embryonic development. I have also characterised the molecular composition of the blood-nerve barrier (BNB), and compared it to the blood-brain barrier using RNA sequencing of purified blood vessels. Finally, we are investigating the role of the INV and BNB in the development of peripheral neuropathy induced by oxaliplatin. While there is currently no treatment, we are trying to propose new therapeutics leads targeting blood vessels.

Prix du meilleur poster

Centre Interdisciplinaire de Recherche en Biologie (CIRB)
Team I.Brunet
UMR7241/INSERM U1050
Collège de France
Paris, France


Centre de Recherche en Cancérologie Immunologie Nantes-Angers
CRCINA, CNRS ERL 6001, INSERM U1232
Université de Nantes
Nantes, France
Kilian Trillet is engineer at « Centre de Recherche Nantes-Angers » in the team SOAP (Signaling in Oncogenesis, Angiogenesis and Permeability) lead by Dr Julie Gavard working on the molecular piracy exerted by tumor cells to survive, how they adapt and remodel their environment, and the signaling mechanisms involved in non-oncogene addiction and loss of vascular homeostasis. In particular, Kilian research domain is focused on investigating the mechanisms of the G protein-coupled Apelin receptor in Glioblastoma Stem-like Cells (GSCs) opening new therapeutic perspectives toward the targeting of cancer stem cells.